On their travels or while shopping online, Taiwanese nationals frequently purchase dietary supplements. The Taiwan Food and Drug Administration warns the general public not to buy or consume dietary supplements with ambiguous labels, a claim of exaggerated efficacy, or unknown provenance, particularly pharmaceuticals that have not been given the go-ahead by regional health authorities. To prevent medicines having prohibited components that might jeopardize other people's health or even violate the law, do not distribute or use medications as gifts to friends or resell them to others.
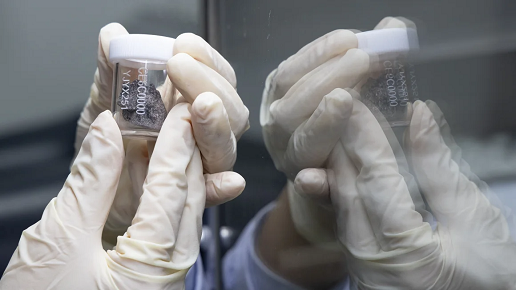
Taiwan Food and Drug Administration cautions the general public not to purchase medications from unreliable sources.Photo reproduced from Taiwan Food and Drug Administration Facebook
According to the Taiwan Food and Drug Administration, customs recently stopped an express shipment of Japanese laxatives that Taiwanese nationals to Japan frequently purchase. However, the contents were green and white capsules, which were inconsistent with the original pink pills. It was discovered to include sibutramine and phenolphthalein after being tested by the Taiwan Food and Drug Administration, and the FDA has long since withdrawn the drug license for products containing these substances. Palpitations, arrhythmias, cardiac arrest, and even death are possible adverse effects of sibutramine. A laxative that might encourage bowel motions is phenolphthalein. Abdominal discomfort, electrolyte imbalance, and cancer are all possible side effects of prolonged usage.
In order to prevent violating the law, the Taiwan Food and Drug Administration also cautions the general public that medications purchased from abroad cannot be openly advertised or sold. According to Article 82 of the Pharmaceutical Affairs Act, any illegally produced or imported counterfeit medications are subject to a fine of no more than NT$100 million and a fixed-term jail sentence of no longer than ten years may be issued. According to Article 83 of the Pharmaceutical Affairs Act, anybody who sells, provides, or displays medications with the intent to sell them without a license faces a fixed-term jail sentence of no more than seven years and a fine of no more than NT$50 million.