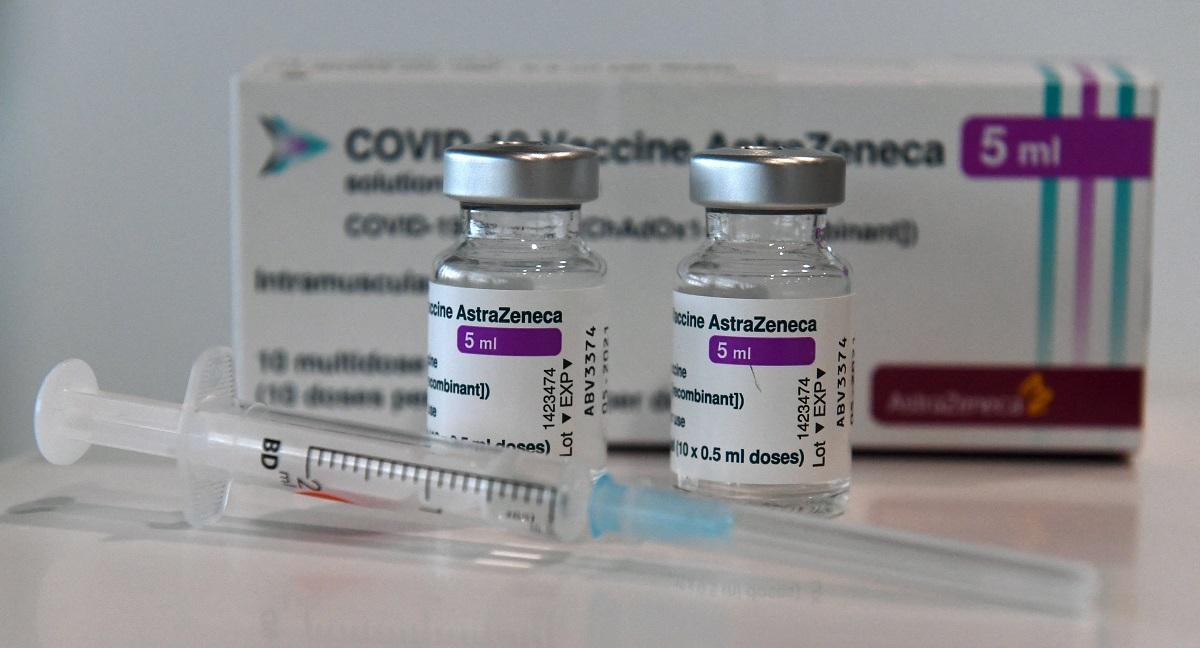
According to INQUIRER.NET, the Department of Health (DOH) said on April 8 that it was temporarily halting the use of the AstraZeneca vaccine on those aged 60 and below, following the recommendation of the Food and Drug Administration (FDA).
The recommendation was due to concerns over recent reports of rare cases of blood clots with low platelets detected in some individuals inoculated with the vaccine in other countries.
FDA Director General Eric Domingo, however, said the suspension was just a precaution.
“I want to emphasize that this temporary suspension does not mean that the vaccine is unsafe or ineffective—it just means that we are taking precautionary measures to ensure the safety of every Filipino,” Domingo said.
More articles: Cities and Towns in the Philippines are starting stricter MECQ
INQUIRER.NET mentions, the Vaccine Expert Panel (VEP) was not consulted regarding this decision, according to panel member and infectious disease expert Dr. Rontgene Solante.
Of the 525,600 AstraZeneca vaccine doses the Philippines received from the COVAX facility on March 4 and March 7, only 31,912 were left and had not been administered as of Thursday, the National Vaccination Operations Center said.
Many of those who received AstraZeneca are health-care workers who are now waiting for their second shot.
“I’m not aware yet if those who received the first dose can’t receive the second dose. [But] they should,” Solante said.
More articles: Zentangle art activity in Miaoli on May 23 and May 29