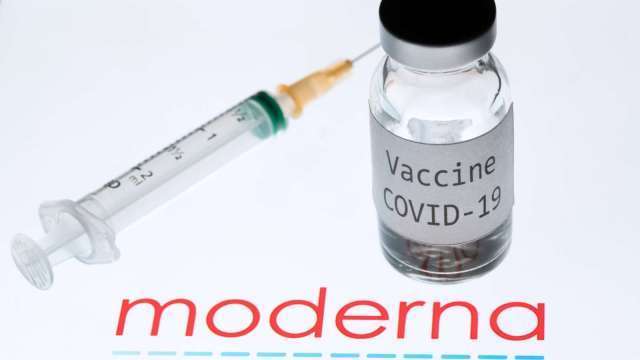
According to philstar GLOBAL, the Philippines approved the COVID-19 vaccine of United States biotech company Moderna for emergency use, making it the seventh coronavirus jab to get clearance in the country.
Food and Drug Administration Director General Eric Domingo said during a news conference that the benefits of administering the vaccine outweighs the risks.
The authorization, which was issued just nine days after local distributor Zuellig Pharma applied, clears Moderna’s jab for individuals aged 18 and older.
The emergency use authorization will only be valid within the duration of the declared public health emergency or upon the issuance of certificate of product registration.
More articles: Hu Shu Fen: a social worker who is always there for new immigrants
Philstar GLOBAL mentions, Moderna is taken in two doses, and has reported an efficacy rate of 94%. Regulators in the US also approved it for emergency use in late 2020.
Philippine Ambassador to the US Jose Manuel Romualdez said Moderna has committed to deliver 200,000 doses of its vaccine by June 15.
The tripartite deal was made between the government, the manufacturer and the private sector. Here, the government will get 13 million doses, while the private sector will get the remaining seven million for its frontline workers.
Government data showed that as of May 3, 309,385 Filipinos are already fully-vaccinated, while 1.69 million have received their first dose.
More articles: Migrant workers arriving in Taiwan are required to complete a 14-day home quarantine and 7-day self-health monitoring procedure